
The d and f orbitals have more complex shapes.
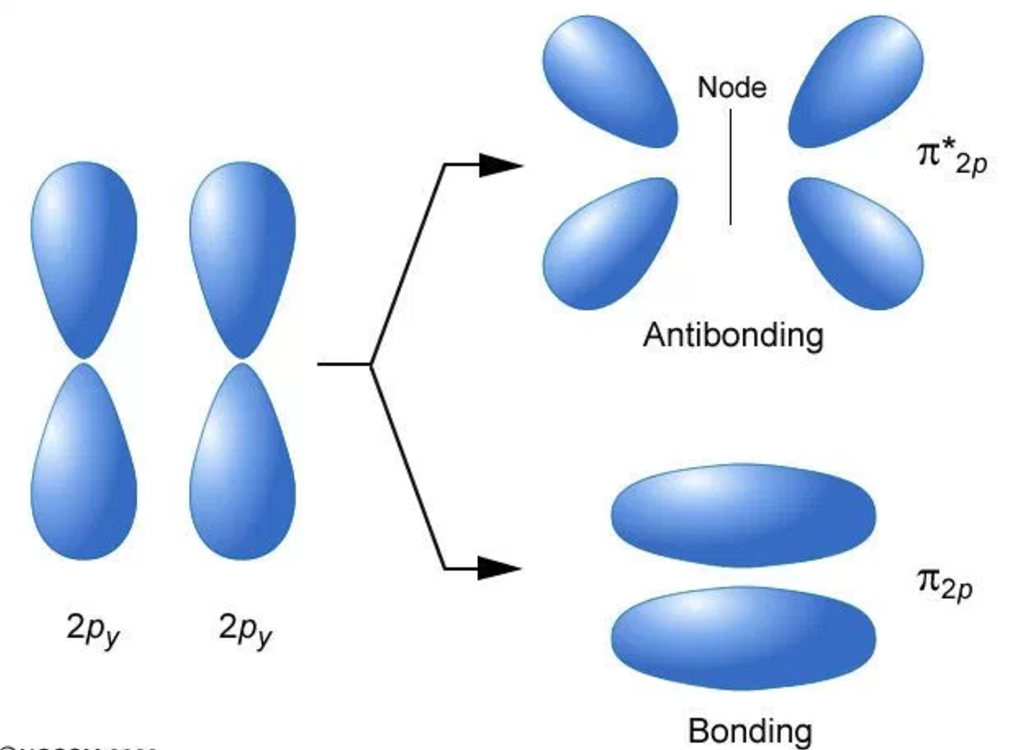
The p orbital is dumbbell shaped and can hold up to six electrons. The s orbital is spherical and hold a maximum of two electrons. Atomic orbitals are labeled as s, p, d, and f sublevels. An atomic orbital can have a maximum of two electrons. The electron density of an atom can be found from the solutions of the Schrodinger equation. It is explained in Heisenberg’s uncertainty principle. It does not explain the exact energy of an electron at a given prompt of time. Quantum mechanics explain the probability of the location of an electron of an atom. The Atomic orbital is a region having the highest probability of finding an electron. What is the difference between Atomic Orbital and Molecular Orbital – Definition, Characteristics, Propertiesģ. The main difference between atomic and molecular orbital is that the electrons in an atomic orbital are influenced by one positive nucleus, while the electrons of a molecular orbital are influenced by the two or more nuclei depending upon the number of atoms in a molecule. Orbitals can hold a maximum of two electrons. Valence bond theory and molecular orbital theory explains the properties of atomic and molecular orbitals, respectively. When these orbitals are overlapped to form molecules through the bonding, the orbitals are called molecular orbitals. Atoms have their own electrons rotating around the nucleus. Orbital is defined as a region where the probability of finding an electron is high. Main Difference – Atomic Orbital vs Molecular Orbital


 0 kommentar(er)
0 kommentar(er)
